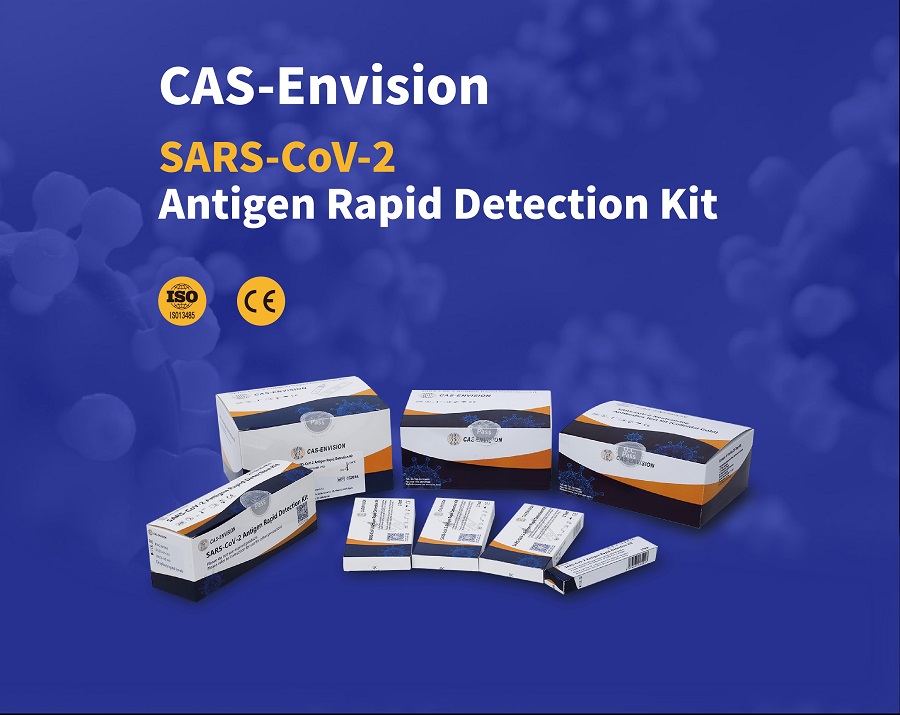
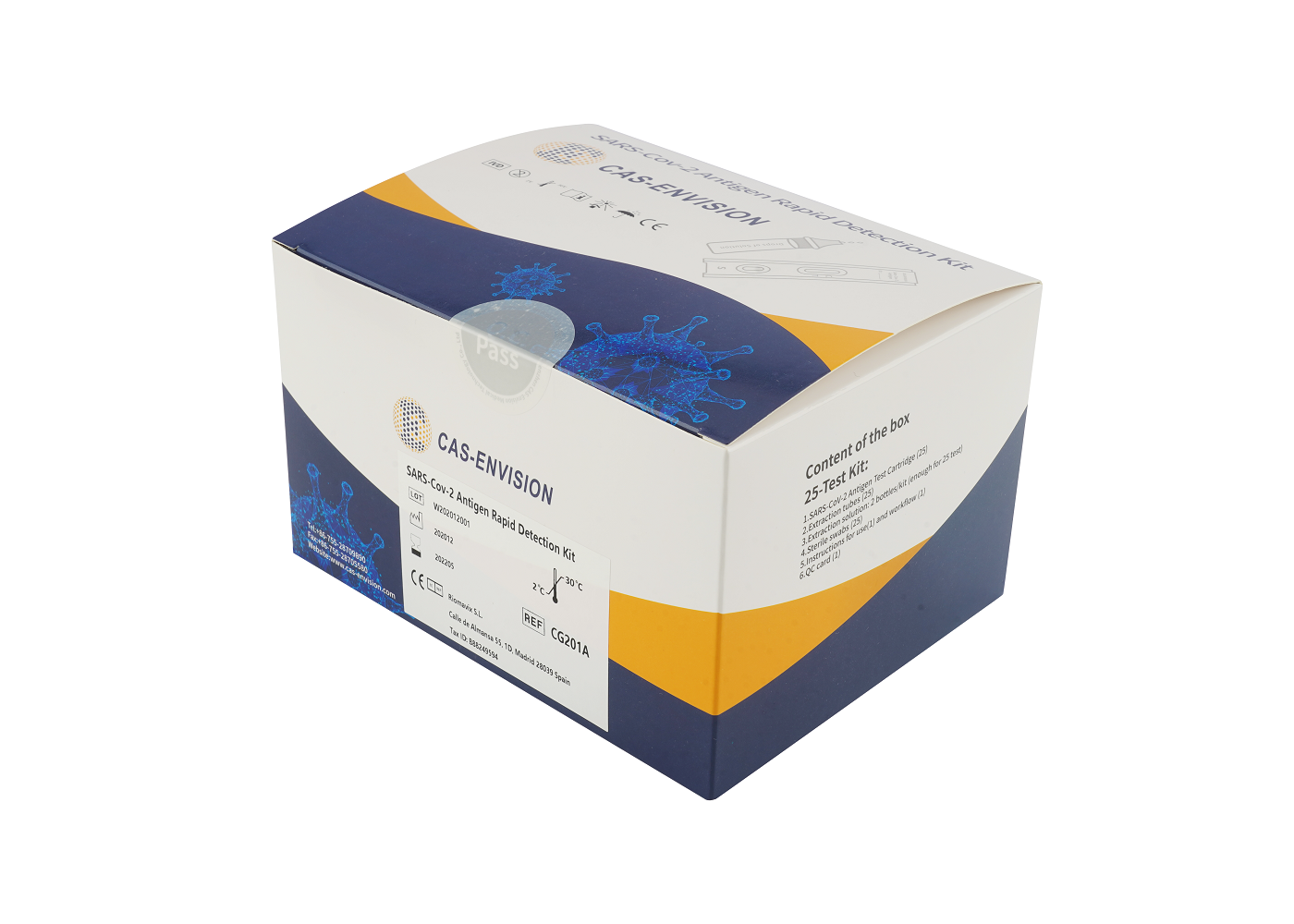
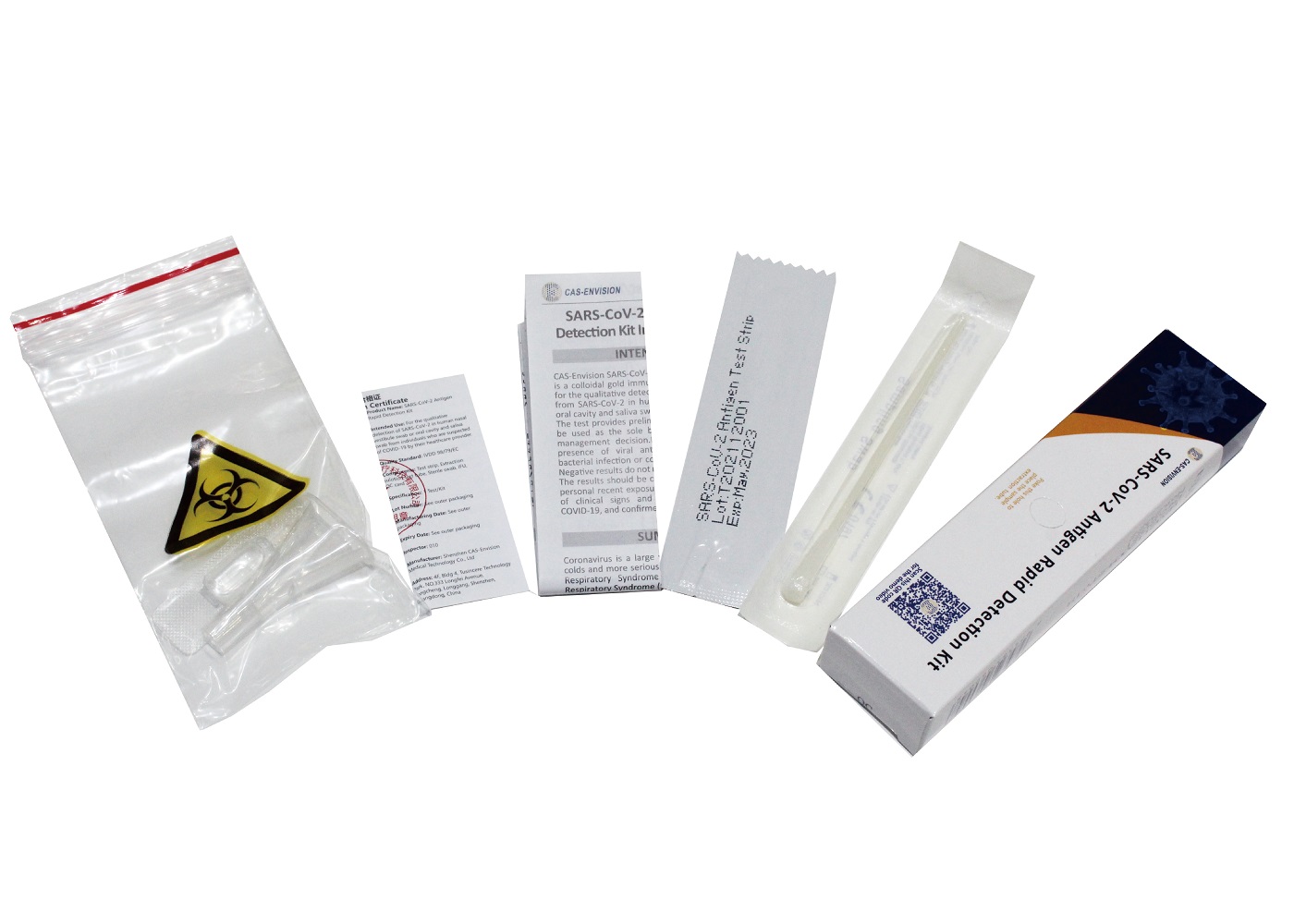
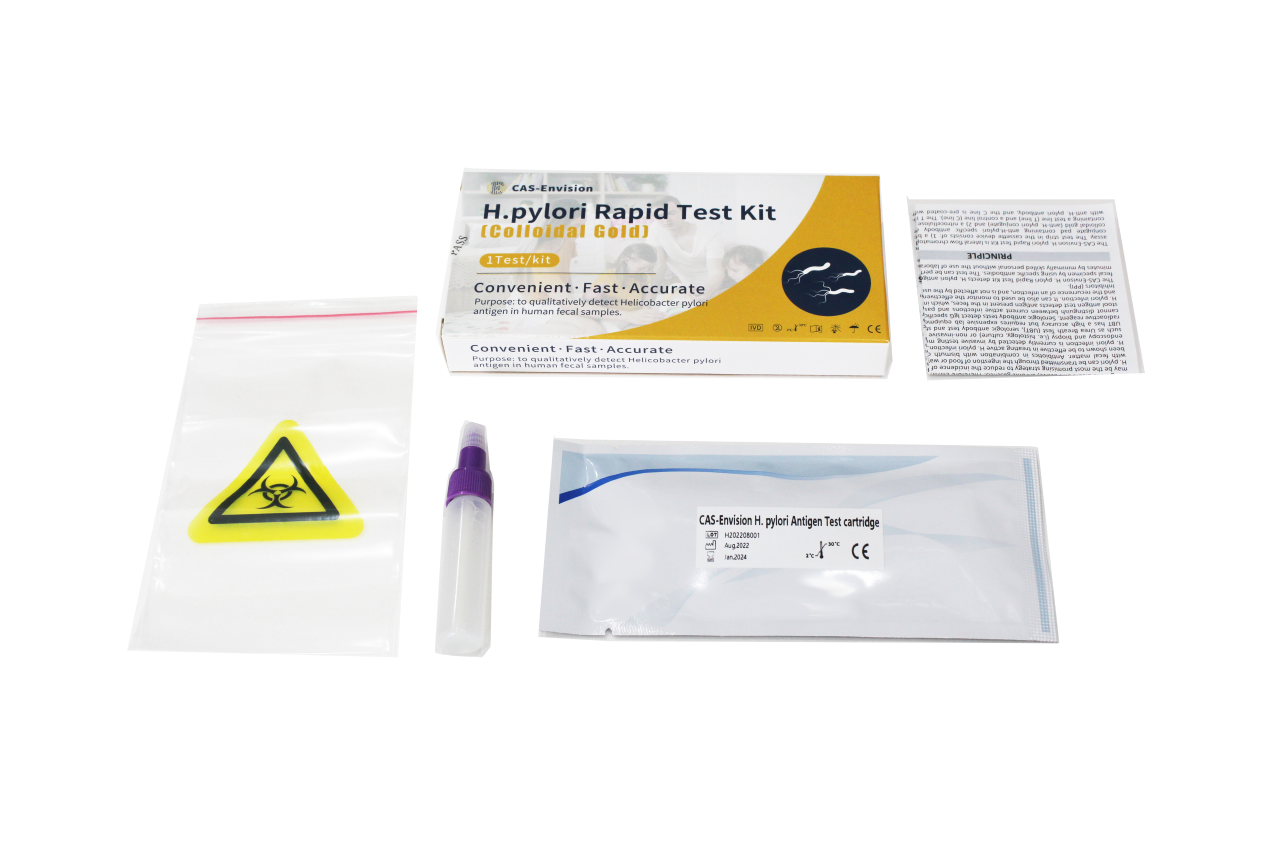
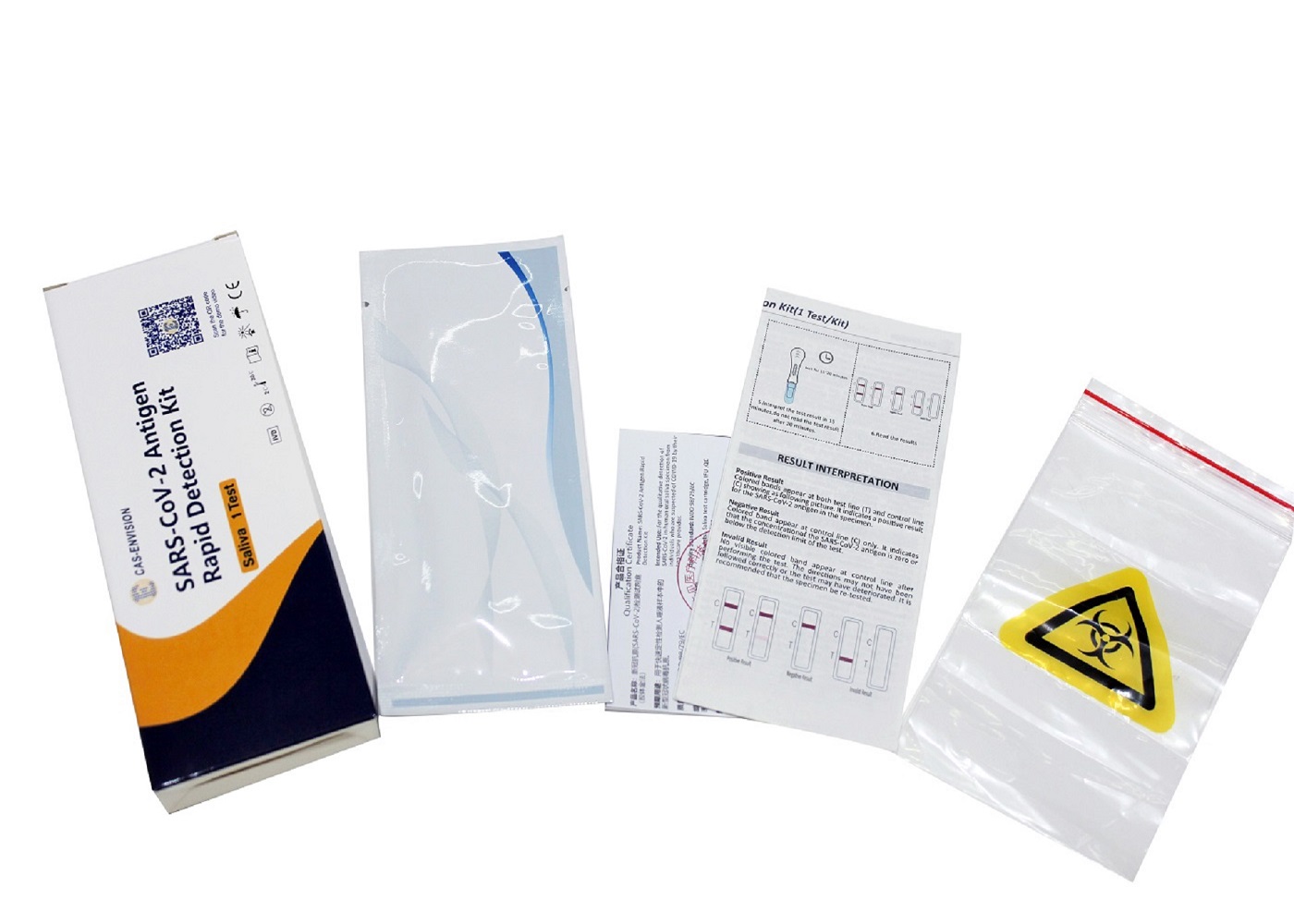
PRODUCT
产品中心
About Us
Shenzhen CAS-Envision Medical Technology Co., Ltd.is a high-tech start-up enterprise held by SIAT (Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences). Relying on first-class talents introduced from overseas, it established “artificial retina” Guangdong innovation team and Shenzhen peacock team and industrialized their technical achievements.

服务中心